Broadening the scope of Baeyer–Villiger monooxygenase activities toward α,β-unsaturated ketones: a promising route to chiral enol-lactones and ene-lactones†
Abstract
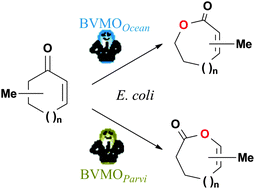
Three regiodivergent Baeyer–Villiger mono-oxygenases (enantioselectively) oxidized a series of cyclic α,β-unsaturated ketones into (chiral) either enol-lactones or ene-lactones. An easy-to-use and efficient biocatalytic process based on a host-microorganism deprived of unwanted activities (knock-out mutant) was developed to enable the exclusive synthesis of unsaturated lactones.

 Please wait while we load your content...
Please wait while we load your content...