Face to face activation of a phenylselenium borane with α,β-unsaturated carbonyl substrates: facile synthesis of C–Se bonds†
Abstract
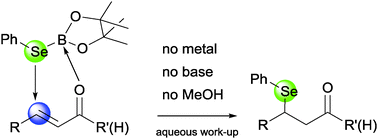
Activated olefins directly react with a phenylselenium borane, at room temperature, without any metal or organocatalytic assistance. Up to 10 examples of β-(phenylseleno) substituted ketones and aldehydes have been prepared and theoretical evidence for the mechanism opens up non-existing pathways to create C–heteroatom bonds as a general tool.

 Please wait while we load your content...
Please wait while we load your content...