Synergistic catalysis: highly diastereoselective benzoxazole addition to Morita–Baylis–Hillman carbonates†
Abstract
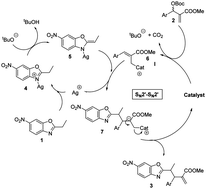
An expedited method has been developed for the diastereoselective synthesis of highly functionalized alkyl-azaarene systems with good yields and high diastereoselectivities (>15 : 1 dr). The methodology includes a synergistic catalysis event involving organometallic (10 mol% AgOAc) activation of an alkyl azaarene and Lewis base (10 mol% DABCO) activation of a Morita–Baylis–Hillman carbonate. The structure and relative configuration of a representative product were confirmed by X-ray analysis.

 Please wait while we load your content...
Please wait while we load your content...