Enantio-differentiation of O-heterocycles using a binol-derived disulfonimide as a chiral solvating agent†
Abstract
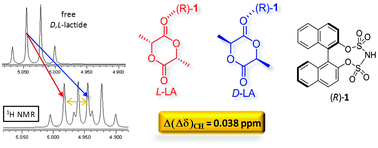
The disulfonimide (R)-1 enables enantio-differentiation of a large scope of chiral O-heterocycles, thanks to the formation of diastereomeric adducts. The underlying H-bond has been investigated by NMR. (R)-1 has been used as a chiral solvating agent (CSA) to determine the stereochemical purity of D/L lactide by 1H NMR.

 Please wait while we load your content...
Please wait while we load your content...