Solvent and electrolyte effects on Ni(PR2NR′2)2-catalyzed electrochemical oxidation of hydrogen†
Abstract
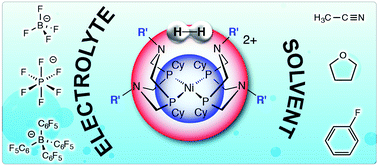
We report solvent and electrolyte effects on the electrocatalytic oxidation of H2 using Ni(PCy2NR′2)2 (R = Bn, tBu) complexes. A turnover frequency of 46 s−1 for Ni(PCy2NBn2)2 was obtained using 0.2 M [nBu4N][BF4] in THF. A turnover frequency of 51 s−1 was observed for Ni(PCy2NtBu2)2 using 0.2 M [nBu4N][B(C6F5)4] in fluorobenzene. These observations, in conjunction with previous studies, indicate nitrile binding inhibits catalysis supported by Ni(PCy2NBn2)2.
- This article is part of the themed collection: Metal-Mediated Transformations of Small Molecules

 Please wait while we load your content...
Please wait while we load your content...