Catalytic metal-free Si–N cross-dehydrocoupling†
Abstract
The metal-free B(C6F5)3 catalyzed dehydrocoupling of hydrosilanes with anilines, carbazoles and indoles is reported. For anilines and carbazoles the reaction proceeds by the liberation of H2 as the sole Si–N coupling byproduct. Indoles react with diphenyl(methyl) hydrosilane to give N-silyl indolines with high diastereoselectivity (d.r. 10 : 1) in excellent yields. A mechanism for this Si–N coupling/hydrogenation sequence is proposed.
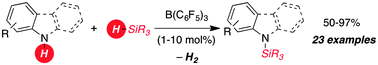

 Please wait while we load your content...
Please wait while we load your content...