Control of cytochrome c redox reactivity through off-pathway modifications in the protein hydrogen-bonding network†
Abstract
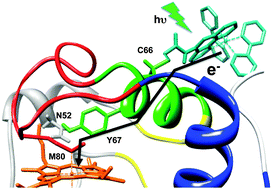
Measurements of photoinduced Fe2+-to-Ru3+ electron transfer (ET), supported by theoretical analysis, demonstrate that mutations off the dominant ET pathways can strongly influence the redox reactivity of cytochrome c. The effects arise from the change in the protein dynamics mediated by the intraprotein hydrogen-bonding network.
- This article is part of the themed collection: 2014 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...