Evidence that steric factors modulate reactivity of tautomeric iron–oxo species in stereospecific alkane C–H hydroxylation†
Abstract
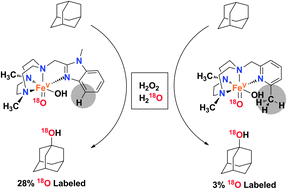
A new iron complex mediates stereospecific hydroxylation of alkyl C–H bonds with hydrogen peroxide, exhibiting excellent efficiency. Isotope labelling studies provide evidence that the relative reactivity of tautomerically related oxo–iron species responsible for the C–H hydroxylation reaction is dominated by steric factors.
- This article is part of the themed collection: Biological oxidation reactions: mechanisms and design of new catalysts

 Please wait while we load your content...
Please wait while we load your content...