Remote chirality control based on the organocatalytic asymmetric Mannich reaction of α-thio acetaldehydes†
Abstract
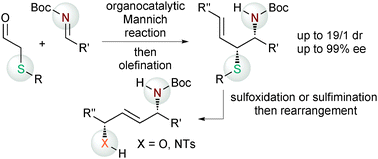
Remote chirality control leading to 1,4-difunctionalized compounds such as 1,4-amino alcohols and 1,4-diamines was achieved by both syn- and anti-selective asymmetric Mannich reactions of α-thio acetaldehydes, the subsequent olefination and the stereospecific 2,3-sigmatropic rearrangement.

 Please wait while we load your content...
Please wait while we load your content...