Zinc-mediated addition of diethyl bromomalonate to alkynes for the cascade reaction towards polysubstituted pyranones and tetracarbonyl derivatives†
Abstract
The zinc-mediated regioselective addition reactions of diethyl bromomalonate and aromatic and aliphatic alkynes were investigated for the synthesis of vinyl malonates. When the vinyl organo-zinc intermediates were reacted with acid chlorides 2H-pyran-2-ones were obtained while the application of oxalyl chloride and an amine led to tetracarbonyl derivatives in a one-pot multi-step reaction sequence.
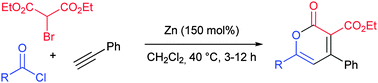

 Please wait while we load your content...
Please wait while we load your content...