Cyclen-based cationic lipids with double hydrophobic tails for efficient gene delivery†
Abstract
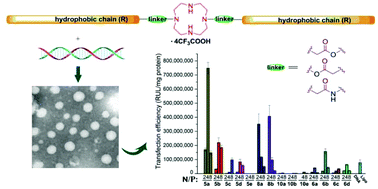
Cationic lipids have been regarded as an important type of non-viral gene vector; to develop novel lipids with high transfection efficiency (TE) and biocompatibility is of great importance. A series of cyclen-based cationic lipids bearing double hydrophobic tails were synthesized herein. To study their structure–activity relationship (SAR), several analogs including the amide-contained double-tailed lipids, lipids containing ester bonds with the reverse direction, and lipids with a single tail were also prepared. Several assays were used to study their interactions with plasmid DNA, and results reveal that these lipids could smoothly condense DNA into nanosized particles. CCK-8-based cell viability assays showed relatively lower cytotoxicity of the lipoplexes compared to commercially available Lipofectamine 2000. In vitro transfection assays exhibited that some of the lipids (5a, 5b and 8b) may give excellent TEs, which were up to 10 times higher than Lipofectamine 2000. SAR of these lipids was studied in detail by investigating the effects of several structural aspects including the chain length and saturation degree, chain numbers, the type of linkage bond, and orientation of ester bonds. The results not only demonstrate that these lipids might be promising non-viral gene vectors, but also afford us clues for further optimization of lipidic gene delivery materials.

 Please wait while we load your content...
Please wait while we load your content...