Colorimetric and ratiometric pH responses by the protonation of phenolate within hemicyanine†
Abstract
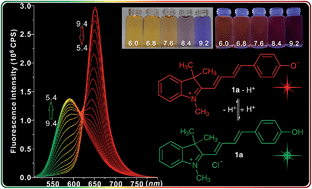
Compared to the nitrogen-reaction based pH optical responsive compounds, oxygen-reaction related pH sensors have attracted less attention. In this paper, hemicyanine based pH probes are designed by establishing the equilibrium between phenolate and phenol, and their reversible absorption and emission responses towards pH are evaluated. The indolium–phenol based tetramethylene hemicyanine (1a) has colorimetric responses at 455 and 578 nm due to the protonating and deprotonating processes; its emission spectra shows ratiometric changes at 594 and 654 nm with large Stokes shifts under acidic (139 nm) and basic conditions (76 nm). The bromide substituent of the hemicyanine (1b) has a lower pKa value compared with unsubstituted hemicyanine (1a), which suggests that adjustable pKa can be achieved by the modification of electron withdrawing groups. The theoretical calculations based on the density functional theory (DFT) were also used to explain the optical properties. Moreover, the in cellulo fluorescence imaging shows that the hemicyanine (1a) can be used for the detection of intracellular pH levels.

 Please wait while we load your content...
Please wait while we load your content...