Block copolymer templated synthesis of PtIr bimetallic nanocatalysts for the formic acid oxidation reaction†
Abstract
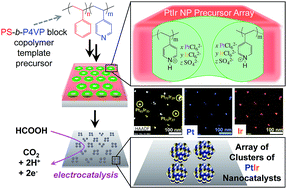
Arrays of PtIr alloy nanoparticle (NP) clusters are synthesized from a method using block copolymer templates, which allows for relatively narrow NP diameter distributions (∼4–13 nm) and uniform intercluster spacing (∼60 or ∼100 nm). Polystyrene-block-poly(4-vinylpyridine) (PS-b-P4VP) block copolymer micelles were used to create thin film templates of NPs with periodic pyridinium-rich domains that are capable of electrostatically loading PtCl62− and IrCl62− anion precursors for the preparation of NP arrays. The composition of PtIr NPs was specified by the ratio of metal anions in a low-pH immersion bath. Formic acid oxidation, studied by cyclic voltammetry, shows that the arrays of clusters of PtIr alloy NPs are highly active catalysts, with mass activity values on par or exceeding current industrial standard catalysts. The uniformity in the NP population in a cluster and the small diameter range established by the block copolymer template permit an estimate of the optimal Pt : Ir ratio for the direct oxidation of formic acid, where, ∼10 nm Pt16Ir84 alloy NPs were the most active with a mass activity of 37 A g−1.


 Please wait while we load your content...
Please wait while we load your content...