pH-dependent nano-capturing of tartaric acid using dendrimers†
Abstract
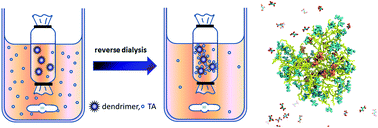
The ability of dendrimers to bind to various target molecules through non-covalent interactions was used to capture water soluble organic reagents, such as tartaric acid (TA), from different matrices, i.e. aqueous solutions and wine samples. The influence of the pH, dendrimer type, generation and feeding concentration on the host–guest complexation of TA was investigated. The maximum binding capacity of TA in aqueous solutions was achieved by amine end-capped dendrimers at pH 5. At extreme pH values of 2 and 11, the binding of TA dropped strikingly, demonstrating the pH-dependency underlying the host–guest interactions. The linear correlation between the maximum binding capacity of TA at pH 5 and the number of primary amine groups on the surface of PAMAM and PPI dendrimers strongly indicated that host–guest complex formation between TA and dendrimers is largely dependent on electrostatic interactions. Molecular simulations confirmed the predominant electrostatic nature of the interactions between TA and the amine end-capped dendrimers and also provided important information on the spatial distribution of TA within the PAMAM G5 dendrimer. All these results designate dendrimers as potential nano-capturing systems for the removal/recovery of TA from complex matrices such as wine, industrial waste or fruit juices.

 Please wait while we load your content...
Please wait while we load your content...