Efficient conversion of primary azides to aldehydes catalyzed by active site variants of myoglobin†
Abstract
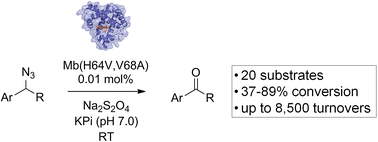
The oxidation of primary azides to aldehydes constitutes a convenient but underdeveloped transformation for which no efficient methods are available. Here, we demonstrate that engineered variants of the hemoprotein myoglobin can catalyze this transformation with high efficiency (up to 8500 turnovers) and selectivity across a range of structurally diverse aryl-substituted primary azides. Mutagenesis of the ‘distal’ histidine residue was particularly effective in enhancing the azide oxidation reactivity of myoglobin, enabling these reactions to proceed in good to excellent yields (37–89%) and to be carried out at a synthetically useful scale. Kinetic isotope effect, isotope labeling, and substrate binding experiments support a mechanism involving heme-catalyzed decomposition of the organic azide followed by alpha hydrogen deprotonation to generate an aldimine which, upon hydrolysis, releases the aldehyde product. This work provides the first example of a biocatalytic azide-to-aldehyde conversion and expands the range of non-native chemical transformations accessible through hemoprotein-mediated catalysis.

 Please wait while we load your content...
Please wait while we load your content...