Intermolecular carbene S–H insertion catalysed by engineered myoglobin-based catalysts†
Abstract
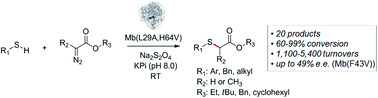
The first example of a biocatalytic strategy for the synthesis of thioethers via an intermolecular carbene S–H insertion reaction is reported. Engineered variants of sperm whale myoglobin were found to efficiently catalyze this C–S bond forming transformation across a diverse set of aryl and alkyl mercaptan substrates and α-diazoester carbene donors, providing high conversions (60–99%) and high numbers of catalytic turnovers (1100–5400). Furthermore, the enantioselectivity of these biocatalysts could be tuned through mutation of amino acid residues within the distal pocket of the hemoprotein, leading to myoglobin variants capable of supporting asymmetric S–H insertions with up to 49% ee. Rearrangement experiments support a mechanism involving the formation of a sulfonium ylide generated upon attack of the thiol substrate to a heme-bound carbene intermediate.

 Please wait while we load your content...
Please wait while we load your content...