Phenyl-guanidine derivatives as potential therapeutic agents for glioblastoma multiforme: catalytic syntheses, cytotoxic effects and DNA affinity†
Abstract
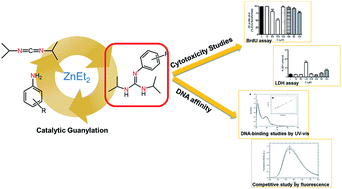
Glioblastoma is a highly malignant form of brain tumor. Current treatment with surgery, temozolomide (TMZ), and radiotherapy only leads to a modest median survival. There is clearly an unmet clinical need for new treatments that are able to arrest the rapid development of the disease through new drugs with antiproliferative activity on glioblastoma cells. In the work described here, several substituted phenyl-guanidine derivatives were developed for application in glioblastoma treatment. The compounds were synthesized by catalytic guanylation reactions and they were fully characterized and assessed for their affinity for DNA by UV titrations and fluorescent intercalator displacement assays. The cytotoxicity levels of the compounds were investigated in the C6 rat glioblastoma cell line by MTT, LDH, and BrdU proliferation assays. Some of the phenyl-guanidine derivatives displayed interesting antitumoral profiles, with a higher potency than the standard drug TMZ in reducing glioblastoma cell proliferation.

 Please wait while we load your content...
Please wait while we load your content...