Correlation of three-liquid-phase equilibria involving ionic liquids†
Abstract
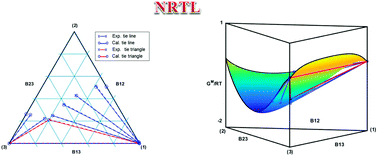
The difficulty in achieving a good thermodynamic description of phase equilibria is finding a model that can be extended to a large variety of chemical families and conditions. This problem worsens in the case of systems containing more than two phases or involving complex compounds such as ionic liquids. However, there are interesting applications that involve multiphasic systems, and the promising features of ionic liquids suggest that they will play an important role in many future processes. In this work, for the first time, the simultaneous correlation of liquid–liquid and liquid–liquid–liquid equilibrium data for ternary systems involving ionic liquids has been carried out. To that end, the phase diagram of the water + [P6 6 6 14][DCA] + hexane system has been determined at 298.15 K and 323.15 K and atmospheric pressure. The importance of this system lies in the possibility of using the surface active ionic liquid to improve surfactant enhanced oil recovery methods. With those and previous measurements, thirteen sets of equilibrium data for water + ionic liquid + oil ternary systems have been correlated. The isoactivity equilibrium condition, using the NRTL model, and some pivotal strategies are proposed to correlate these complex systems. Good agreement has been found between experimental and calculated data in all the regions (one triphasic and two biphasic) of the diagrams. The geometric aspects related to the Gibbs energy of mixing function obtained using the model, together with the minor common tangent plane equilibrium condition, are valuable tools to check the consistency of the obtained correlation results.


 Please wait while we load your content...
Please wait while we load your content...