Direct use of allylic alcohols and allylic amines in palladium-catalyzed allylic amination†
Abstract
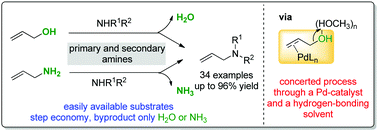
Allylic alcohols and allylic amines were directly utilized in a Pd-catalyzed hydrogen-bond-activated allylic amination under mild reaction conditions in the absence of any additives. The cooperative action of a Pd-catalyst and a hydrogen-bonding solvent is most likely responsible for its high reactivity. The catalytic system is compatible with a variety of functional groups and can be used to prepare a wide range of linear allylic amines in good to excellent yields. Furthermore, this methodology can be easily applied to the one-step synthesis of two drugs, cinnarizine and naftifine, on a gram scale.


 Please wait while we load your content...
Please wait while we load your content...