Phosphonate-stabilized silver nanoparticles: one-step synthesis and monolayer assembly†
Abstract
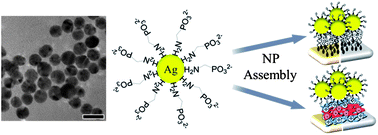
The first case of phosphonic acid terminated, environmentally friendly silver nanoparticles (NPs) is described. The NPs are produced by a simple one-step synthesis using commercially available reagents, furnishing stable, rather monodisperse, size-controlled, aqueous-based Ag NPs, stabilized by aminomethylene phosphonic acid (AMP) molecules. In this synthesis the commercial reagent ethylenediamine tetra(methylene phosphonic acid) (EDTMP) serves as a reducing agent for Ag+ ions, while its oxidation product AMP is the stabilizer of the generated Ag NPs. The negatively charged, phosphonate-stabilized NPs were characterized by UV-vis spectroscopy, transmission electron microscopy (TEM), and X-ray photoelectron spectroscopy (XPS). Variation of the EDTMP/AgNO3 molar ratio enables simple and efficient control of the average particle diameter in the range ∼5.5 to 15 nm. The AMP-stabilized Ag NPs are stable in water under an inert atmosphere for at least 2 months with no observed aggregation. Self-assembled layers of phosphonate-coated Ag NPs were prepared on substrates primed with positively charged molecular self-assembled monolayers (SAMs) or with polyelectrolyte (PE) layers. The NP films were studied by UV-vis spectroscopy, polarization modulation Fourier-transform infrared reflection-absorption spectroscopy (PM-IRRAS), and high-resolution scanning electron microscopy (HRSEM). While NP monolayers commonly undergo extensive aggregation upon drying, the present phosphonate-stabilized Ag NP monolayers display homogeneously dispersed, mostly isolated NPs over large areas after adsorption and drying. The NP distribution and degree of aggregation can be modulated by the substrate type (gold, glass), the colloid solution pH, the nature of the primer layer (charged molecules, PEs), and the surface charge density. Phosphonate-terminated Ag NPs provide unique physical and chemical properties, including a negatively charged surface in a wide pH range, long-term stability, size control, and the possibility of participating in electrostatic and coordination binding processes.

 Please wait while we load your content...
Please wait while we load your content...