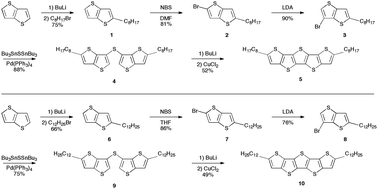
Recent progress in thienoacene development has produced numerous high performance, air-stable organic semiconductor materials. Pentathienoacene (T5) thin film devices have shown poor performance despite promising computational studies, likely due to high mobility anisotropy and poor film morphology. 2,6-Bis-alkyl-pentathienoacene has been synthesized to enable solution-processing routes to better microstructures of T5-based devices. Soluble side groups are introduced to thieno[3,2-b]thiophene precursors through deprotonation at α-positions. Introduction of the sulfur bridge was achieved by Pd-catalyzed coupling reaction with bis(tri-n-butyltin)sulfide (Bu3SnSSnBu3), followed by final ring closure through oxidative coupling with CuCl2. This method achieves higher purity and higher yield than sulfide-quenched Li–Br exchange. UV-vis and fluorescence emission spectra show a bathochromic shift of ≈10 nm, indicating the introduction of alkyl chains decreases the HOMO–LUMO gap. X-ray analysis yields unit cells for 2,6-bis-octyl and 2,6-bis-dodecyl substituted T5s (C8-T5 and C12-T5, respectively). C8-T5 grows orthorhombic crystals with lattice parameters a = 1.15 nm, b = 0.43 nm and c = 3.05 nm; C12-T5 grows monoclinic crystals (c-unique) with unit cell with parameters a = 1.10 nm, b = 0.42 nm, c = 3.89 nm and γ = 92.9°. Both materials grow large (>50 μm), faceted, single crystals that are microscopically composed of alternating layers of semiconducting cores and insulating substitutions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...