Smectic liquid crystals with ‘de Vries-like’ properties are characterized by a maximum layer contraction of ≤1% upon transition from the non-tilted SmA phase to the tilted SmC phase. To expand the current library of ‘de Vries-like’ liquid crystals, we have developed a rational design strategy based on a concept of frustration between two structural elements, one promoting the formation of a SmA phase (chloro-terminated side-chain) and another promoting the formation of a SmC phase (siloxane-terminated side-chain). In this paper, we show that one can tune this apparent frustration—and further improve de Vries-like properties—by substituting the 2-phenylpyrimidine core in our first-generation siloxane-terminated mesogens with one of three cores known to be stronger SmC-promoting elements: 6-phenylpyridazine, 2-phenylpyridine and 2-phenylthiadiazole. We also address a fundamental design flaw of siloxane-terminated mesogens, i.e., the hydrolytic instability of siloxane oligomers, by substituting the siloxane end-group with a chemically inert carbosilane end-group. As a result of this study, we found a carbosilane-terminated 2-phenylthiadiazole mesogen that forms a SmC phase at room temperature with de Vries-like properties that are comparable to those of bona fide de Vries-like liquid crystals.
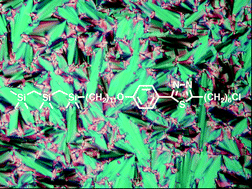
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
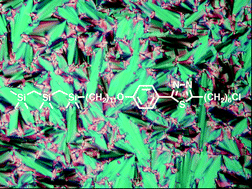

 Please wait while we load your content...
Please wait while we load your content...