Porphyrin oriented self-assembled nanostructures for efficient exciton dissociation in high-performing organic photovoltaics†
Abstract
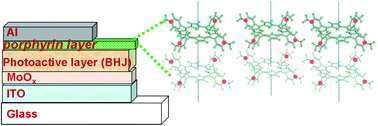
Herein we report on enhanced organic solar cell performance through the incorporation of cathode interfacial layers consisting of self-organized porphyrin nanostructures with a face-on configuration. In particular, a water/methanol-soluble porphyrin molecule, the free base meso-tetrakis(1-methylpyridinium-4-yl)porphyrin chloride, is employed as a novel cathode interlayer in bulk heterojunction organic photovoltaics. It is demonstrated that the self-organization of this porphyrin compound into aggregates in which molecules adopt a face-to-face orientation parallel to the organic semiconducting substrate induces a large local interfacial electric field that results in a significant enhancement of exciton dissociation. Consequently, enhanced photocurrent and open circuit voltage were obtained resulting in overall device efficiency improvement in organic photovoltaics based on bulk heterojunction mixtures of different polymeric donors and fullerene acceptors, regardless of the specific combination of donor–acceptor employed. To highlight the impact of molecular orientation a second porphyrin compound, the Zn-metallated meso-tetrakis(1-methylpyridinium-4-yl)porphyrin chloride, was also studied and it was found that it forms aggregates with an edge-to-edge molecular configuration inducing a smaller increase in the device performance.

 Please wait while we load your content...
Please wait while we load your content...