Reversible hydrogen storage in Mg(BH4)2/carbon nanocomposites†
Abstract
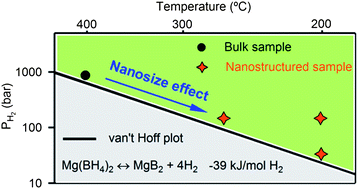
Mg(BH4)2 exhibits a high hydrogen content of 14.9 wt% and thermodynamic stability in the overall decomposition reaction that corresponds to hydrogen desorption at around room temperature. However, the potential applications in hydrogen storage are restricted by high kinetic barriers. In this study, we show the synthesis of Mg(BH4)2/carbon nanocomposites by

 Please wait while we load your content...
Please wait while we load your content...