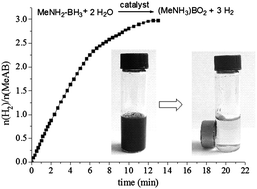
Well dispersed magnetically recyclable trimetallic core–shell Ag@CoNi nanoparticles (NPs) supported on graphene have been synthesized via a facile one-step in situ procedure using methylamine borane (MeAB) as the reducing agent. The as-synthesized NPs exhibit much higher catalytic activities for hydrolytic dehydrogenation of ammonia borane (AB) than the monometallic, bimetallic, trimetallic alloy (AgCoNi/graphene), and graphene free (Ag@CoNi) counterparts. Moreover, compared with NaBH4 and AB, the weaker reducing agent MeAB has much better control during the synthesis of the graphene supported Ag@CoNi NPs, which resulted in the highest catalytic activity. Kinetic studies indicate that the catalytic hydrolysis of AB by the Ag@CoNi/graphene NPs is first order, with the activation energy measured to be 36.15 kJ mol−1. Furthermore, the as-prepared NPs exert good catalytic activities and recycle stabilities towards the hydrolysis of AB and MeAB. Hence, this general method indicates that MeAB can be used as both a potential hydrogen storage material and an efficient reducing agent, and can be easily extended to facile preparation of other graphene supported multi-metal NPs.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...