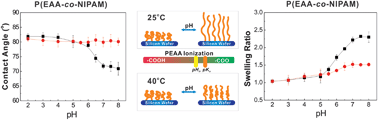
We report on weak polyacid brushes with highly tunable pH and temperature response characteristics. This was achieved by synthesizing a series of homo- and copolymers which contain 2-alkylacrylic acids (aAAs) of increased hydrophobicity, i.e. acrylic acid (AA), methacrylic acid (MAA), or 2-ethylacrylic acid (EAA), using surface-initiated reversible addition–fragmentation chain transfer (SI-RAFT) polymerization. As revealed by contact angle measurements, in situ ellipsometry and AFM studies of brush swelling, the pH-response of PAA and PMAA brushes was similar, with brushes remaining highly swollen (swelling ratio 2.5–3.0) at low pH values. The PEAA brush, however, was unique as it showed low degrees of water uptake (<10%) at pH < 5 due to insolubility of this polyelectrolyte in acidic solutions, moderate swelling at high pH and relatively high (>70°) contact angles in the entire pH region from 2 to 8. Copolymer brushes of aAAs with N-isopropylacrylamide (NIPAM), denoted as P(AA-co-NIPAM), P(MAA-co-NIPAM) and P(EAA-co-NIPAM), demonstrated dual pH and temperature response, which was strongly dependent on the type of aAA co-monomer. P(AA-co-NIPAM) and P(MAA-co-NIPAM) brushes underwent large-amplitude pH-induced changes in brush swelling and water contact angle in the range of pH from 3 to 6, and were only weakly responsive to temperature in the transition region. In contrast, more hydrophobic P(EAA-co-NIPAM) brushes demonstrated both pH and temperature responses at physiologically relevant neutral/basic pH values even when the content of EAA units in the copolymer was as high as ∼50%. We discuss the role of inter- and intra-molecular hydrogen bonding and monomer hydrophobicity and ionization (quantified by FTIR) in determining pH ranges for brush response. These findings might enable control of molecular/cellular adhesion and flow at interfaces potentially useful in microfluidic and biomedical applications.

 Please wait while we load your content...
Please wait while we load your content...