Fragility and glass transition temperature of polymer confined under isobaric and isochoric conditions
Abstract
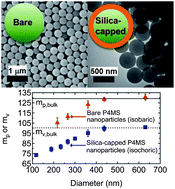
It is now well established that the glass transition temperature (Tg) of polymers can deviate substantially from the bulk with nanoscale confinement. Understanding the impact of confinement on the Tg of polymers is important but it does not provide information about the temperature dependence of the cooperative segmental dynamics near the Tg, a phenomenon commonly referred to as the dynamic fragility. Here, we measure the dynamic fragility index (m) as well as the Tg of confined poly(4-methylstyrene) (P4MS) under isobaric and isochoric conditions. We accomplish this via variable cooling rate differential scanning calorimetry (DSC) studies on aqueous-suspended and silica-capped P4MS nanoparticles. We observe that both the isobaric (mp) and isochoric (mv) fragilities decrease with confinement. However, Tg decreases and remains constant with isobaric and isochoric confinement, respectively. The importance of interfaces in the observed trends as well as comparisons to prior studies is discussed.
- This article is part of the themed collection: Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...