Highly enantioselective S–H bond insertion cooperatively catalyzed by dirhodium complexes and chiral spiro phosphoric acids†
Abstract
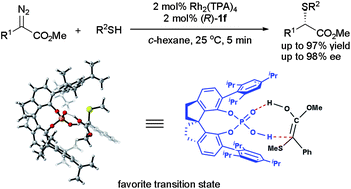
The first highly enantioselective S–H bond insertion reaction was developed by cooperative catalysis of dirhodium(II) carboxylates and chiral spiro phosphoric acids (SPAs) under mild and neutral reaction conditions with fast reaction rates, high yields (77–97% yields), and excellent enantioselectivities (up to 98% ee). The catalytic S–H bond insertion reaction provides a highly efficient method for the synthesis of chiral sulfur-containing compounds and advances the synthesis of a chiral sulfur-containing drug (S)-Eflucimibe. A systematic 31P NMR study revealed that no ligand exchange between dirhodium(II) carboxylates and SPAs occurred in the reaction. The distinct behaviors of cooperative catalysts Rh2(TPA)4/(R)-1a and the prepared complex Rh2(R-1a)4 observed by in situ FT-IR spectroscopy excluded the feasibility of Rh2(R-SPA)4 being the real catalyst. DFT calculations showed that the activation barrier in the proton shift step became remarkably low as promoted by SPAs. Based on the experimental results and the calculations, the SPA was proposed as a chiral proton shuttle for the proton shift in reaction. Additionally, the single crystal structures of several SPAs were measured and used to rationalize the configurations of the S–H insertion products obtained in the reactions. The rigid and crowded environment around the SPAs ensures the high enantioselectivity in the S–H bond insertion reaction.

 Please wait while we load your content...
Please wait while we load your content...