Application of diazene-directed fragment assembly to the total synthesis and stereochemical assignment of (+)-desmethyl-meso-chimonanthine and related heterodimeric alkaloids†
Abstract
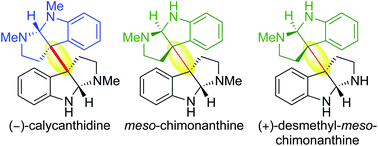
We describe the first application of our methodology for heterodimerization via diazene fragmentation towards the total synthesis of (−)-calycanthidine, meso-chimonanthine, and (+)-desmethyl-meso-chimonanthine. Our syntheses of these alkaloids feature an improved route to C3a-aminocyclotryptamines, an enhanced method for sulfamide synthesis and oxidation, in addition to a late-stage diversification leading to the first enantioselective total synthesis of (+)-desmethyl-meso-chimonanthine and its unambiguous stereochemical assignment. This versatile strategy for directed assembly of heterodimeric cyclotryptamine alkaloids has broad implications for the controlled synthesis of higher order derivatives with related substructures.

 Please wait while we load your content...
Please wait while we load your content...