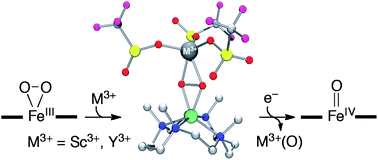
Redox-inactive metal ions that function as Lewis acids play pivotal roles in modulating reactivities of oxygen-containing metal complexes in a variety of biological and biomimetic reactions, including dioxygen activation/formation and functionalization of organic substrates. Mononuclear nonheme iron(III)–peroxo species are invoked as active oxygen intermediates in the catalytic cycles of dioxygen activation by nonheme iron enzymes and their biomimetic compounds. Here, we report mononuclear nonheme iron(III)–peroxo complexes binding redox-inactive metal ions, [(TMC)FeIII(O2)]+–M3+ (M3+ = Sc3+ and Y3+; TMC = 1,4,8,11-tetramethyl-1,4,8,11-tetraazacyclotetradecane), which are characterized spectroscopically as a ‘side-on’ iron(III)–peroxo complex binding a redox-inactive metal ion, (TMC)FeIII–(μ,η2:η2-O2)–M3+ (2–M). While an iron(III)–peroxo complex, [(TMC)FeIII(O2)]+, does not react with electron donors (e.g., ferrocene), one-electron reduction of the iron(III)–peroxo complexes binding redox-inactive metal ions occurs readily upon addition of electron donors, resulting in the generation of an iron(IV)–oxo complex, [(TMC)FeIV(O)]2+ (4), via heterolytic O–O bond cleavage of the peroxide ligand. The rates of the conversion of 2–M to 4 are found to depend on the Lewis acidity of the redox-inactive metal ions and the oxidation potential of the electron donors. We have also determined the fundamental electron-transfer properties of 2–M, such as the reduction potential and the reorganization energy in electron-transfer reaction. Based on the results presented herein, we have proposed a mechanism for the reactions of 2–M and electron donors; the reduction of 2–M to the reduced species, (TMC)FeII–(O2)–M3+ (2′–M), is the rate-determining step, followed by heterolytic O–O bond cleavage of the reduced species to form 4. The present results provide a biomimetic example demonstrating that redox-inactive metal ions bound to an iron(III)–peroxo intermediate play a significant role in activating the peroxide O–O bond to form a high-valent iron(IV)–oxo species.

 Please wait while we load your content...
Please wait while we load your content...