Toward a more step-economical and scalable synthesis of spongistatin 1 to facilitate cancer drug development efforts†
Abstract
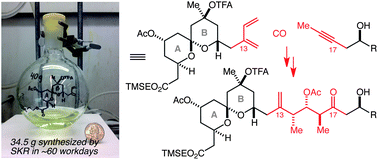
An efficient, step-economical, and scalable synthesis of a diene-bearing AB spiroketal fragment of spongistatin 1, and a demonstration of its efficient coupling to an

 Please wait while we load your content...
Please wait while we load your content...