Asymmetric NHC-catalyzed synthesis of α-fluoroamides from readily accessible α-fluoroenals†
Abstract
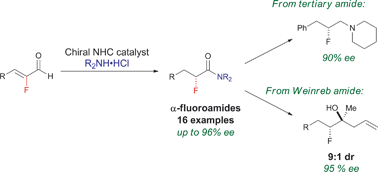
The scope of the NHC-redox amidation has been expanded to include a variety of α,β-unsaturated aldehydes, including α-fluoro α,β-unsaturated aldehydes which give rise to enantioenriched α-fluoroamides in good to excellent yield and enantioselectivity (up to 97% ee). Enantioenriched amines may be elaborated to either diastereomer of the product in high diastereoselectivity (up to 99 : 1). Functionalization of the amide products to amines and fluorohydrins is also demonstrated with retention of enantioenrichment at the fluorine stereocenter.

 Please wait while we load your content...
Please wait while we load your content...