Redox-active, near-infrared dyes based on ‘Nindigo’ (indigo-N,N′-diarylimine) boron chelate complexes†
Abstract
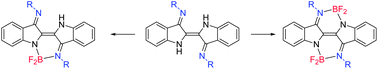
Reactions of indigo-N,N′-diarylimine (‘Nindigo’) derivatives with BF3·Et2O give mono- or bis-BF2 chelate complexes 2 or 3 respectively. The product distribution between 2 and 3 is sensitive to the auxiliary base and

 Please wait while we load your content...
Please wait while we load your content...