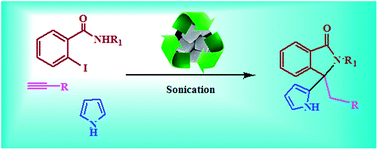
Nanodomain cubic cuprous oxide as reusable catalyst in one-pot synthesis of 3-alkyl/aryl-3-(pyrrole-2-yl/indole-3-yl)-2-phenyl-2,3-dihydro-isoindolinones in aqueous medium†
Abstract
An environmentally benign one-pot protocol has been developed for the syntheses of 3-alkyl/aryl-3-(pyrrole/indole-2/3-yl)-2-phenyl-2,3-dihydro-isoindolinones via a multi-component one-pot reaction involving 2-iodo-N-phenylbenzamides, terminal alkyne and substituted indoles/pyrroles in aqueous medium using cubic cuprous oxide nanoparticles as catalyst. It involves domino Sonogashira-5-exo-dig-cyclization followed by regioselective nucleophilic addition of indoles or pyrroles, in aqueous medium without using any surfactants or additional ligands.

 Please wait while we load your content...
Please wait while we load your content...