Design of nanostructured cadmium tantalate and niobate and their photocatalytic properties†
Abstract
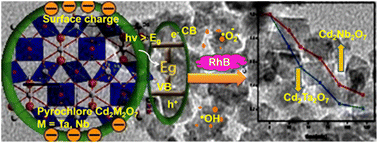
In the present study a template-free hydrothermal method under optimal alkaline conditions and suitable temperature leads to growth of nanocubes (∼15 nm edge) of cadmium tantalate and cadmium niobate which show significantly higher (nearly seven times) photocatalytic activity compared to that obtained by the same oxides by the solid state method. The above synthesis is carried out at much lower temperature (600 °C) than the solid state method (1000 °C). The evolution of the nanostructures as a function of reaction time (hydrothermal synthesis) was followed by microscopy and the optimal time of 48 h leads to oxides with high photocatalytic efficiency. Nanostructures with cuboidal morphology show high surface area (57 m2 g−1) and highly negative surface charge and this leads to enhanced adsorption of the cationic dye (Rhodamine B). These nanoparticles are highly stable under UV light irradiation for a long period of time and retain the photocatalytic efficiency.

 Please wait while we load your content...
Please wait while we load your content...