High stability pyrolyzed vitamin B12 as a non-precious metal catalyst of oxygen reduction reaction in microbial fuel cells
Abstract
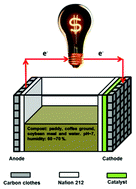
Non-precious metal catalysts are favored for use in microbial fuel cells (MFCs) as they catalyze the oxygen reduction reaction (ORR) at the cathode and are low-cost. In this work, pyrolyzed Vitamin B12 (py-B12/C), supported by carbon black, was utilized as the cathode catalyst in solid phase MFC. The py-B12/C has the highest proportion of quaternary N-type nitrogen than pristine B12, which promotes ORR activity during the pyrolysis at 700 °C, at which temperature the ORR ability is maximized. Py-B12/C has an electron transfer number of 3.85 in O2-saturated 0.05 M phosphate buffered saline (PBS) solution, which is very close to that of Pt/C, which is 3.89. Steady potential tests of py-B12/C and Pt/C reveal that the former does not decay but the latter decays by 24% in 100 h. According to an MFC test, Pt/C has a higher voltage initially but this rapidly falls to zero after ten days owing to the blocking effect of OHads species on Pt surface. Notably, an MFC using py-B12/C exhibits excellent power density of 22.6 mW m−2 and an operational period of more than 30 days, which is longer than that using Pt/C.

 Please wait while we load your content...
Please wait while we load your content...