Novel graphene oxide/manganese oxide nanocomposites†
Abstract
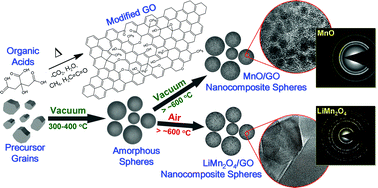
A new synthesis method of obtaining nanocomposites, consisting of manganese oxides nanoparticles embedded in carbonaceous matrix, is reported. The method is based on thermal processing of precursor consisting of lithium and manganese salts mixed with citric and acetic acids. The nanocomposite morphology, composition and structure can be tuned by selecting specific thermal processing routes (e.g. pressure, temperature, time, etc.). For instance, foam or microspheres morphology can be obtained by heating the precursor in a vacuum at moderately low temperatures (ca. 300–400 °C). Similarly, depending on the ambient pressure during heating above the recrystallization temperature (ca. 400–450 °C), the forming nanocomposite will consist of either MnO or LiMn2O4 nanoparticles, i.e. materials which are of importance for lithium-ion batteries as anodes and cathodes, respectively. Finally, the structure of the carbonaceous matrix can be tuned primarily by controlling the annealing temperature. For instance, annealing in the temperature range of about 600–800 °C can lead to the formation of graphene-related structures, such as modified graphene oxide. We used this method to produce example nanocomposites, performed their detailed characterization and proposed a mechanism to explain their formation.

 Please wait while we load your content...
Please wait while we load your content...