Novel cationic fullerene derivatized s-triazine scaffolds as photoinduced DNA cleavage agents: design, synthesis, biological evaluation and computational investigation
Abstract
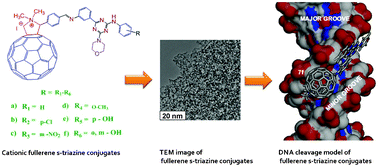
A series of novel cationic fullerene (C60) derivatives, bearing substituted s-triazine moiety as a side arm, synthesized by using the 1,3 dipolar cycloaddition reaction of C60 with azomethine ylides generated from the corresponding Schiff bases of substituted s-triazine is reported. All the synthesized compounds were characterized by elemental analysis, FT-IR, 1H NMR, 13C NMR and ESI-MS. The compounds 7a, 7d, 7e and 7f cleaved the supercoiled pBR322 DNA into nicked form efficiently upon visible light irradiation in the presence of NADH. The photoinduced superoxide radical and hydroxyl radical generated may act as molecular species causing the DNA scission. Further, the interaction of synthesized molecules with pBR322 plasmid DNA was investigated using computational approaches.

 Please wait while we load your content...
Please wait while we load your content...