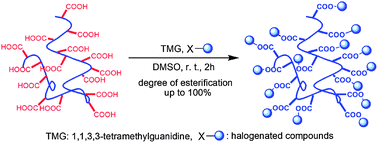
Esterifications of poly(methacrylic acid) (PMAA) and poly(acrylic acid) (PAA) have been investigated with a wide variety of halogenated compounds such as iodide, bromide, and chloride using 1,1,3,3-tetramethylguanidine as a promoter. The results demonstrate that the poly(meth)acrylates can be obtained with an excellent degree of esterification at room temperature. The influence of solvent, reaction conditions, and halogenated compounds on the esterification reaction was examined. It was found that polar solvents, such as dimethyl sulfoxide (DMSO) and N,N-dimethylformamide (DMF), are favorable for the esterification and high degree of esterification can be achieved in a short time. Moreover, the esterification reaction of PMAA has been successfully performed in an aqueous solution of DMSO, which indicates that the solvent does not necessarily have to be dried for this reaction. Primary and secondary halogenated compounds can successfully react with PMAA or PAA, while tertiary halogenated compounds fail to react. In addition, combining this esterification reaction with atom transfer radical polymerization (ATRP), macromonomers were conveniently prepared by the reaction of halogen-capped polymers with methacrylic acid under mild conditions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...