Excited state dynamics of the photoconvertible fluorescent protein Kaede revealed by ultrafast spectroscopy†
Abstract
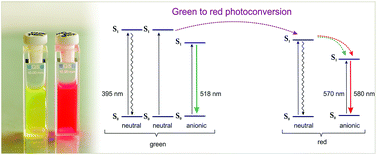
The ultrafast excited state dynamics of the fluorescent protein Kaede has been investigated by employing time resolved fluorescence and transient absorption. Upon irradiation of its neutral state, the protein undergoes an efficient conversion to a state that fluoresces at longer wavelengths. The molecular basis of the photoconversion involves an expansion of the chromophore π-conjugation by formal β-elimination but details of the reaction pathway remain subject to debate. Based on the kinetics observed in experiments on the protein sample in both H2O and D2O buffers, we suggest that a light-initiated cleavage mechanism (20 ps) could take place, forming the neutral red state in which the red chromophore resides. Excitation of the neutral red form results in the formation of the red anionic species via two Förster resonance energy transfer (FRET) channels. FRET between red neutral and red anionic forms occurs within the tetramer with time constants of 13.4 ps and 210 ps. In contrast to literature proposals no ESPT was observed.
- This article is part of the themed collection: International Conference on Photochemistry 2013 (ICP2013) keynote lectures

 Please wait while we load your content...
Please wait while we load your content...