Enantioselective Michael addition of 1,3-dicarbonyl compounds to a nitroalkene catalyzed by chiral squaramides – a key step in the synthesis of pregabalin†
Abstract
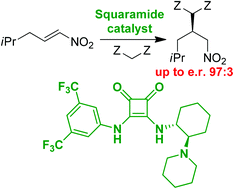
Asymmetric organocatalytic 1,4-additions provide access to a large number of biologically relevant compounds. Chiral squaramides efficiently catalyse enantioselective Michael addition of 1,3-dicarbonyl compounds to aliphatic nitroalkenes. The resulting γ-nitro carboxylic derivatives were obtained in high yields and in high enantiomeric purities. Quantum chemical calculations helped us to devise a transition state model, which explains the observed stereochemical course of the addition. The best results were obtained with Meldrum's acid as a donor, with which enantiomeric purity of the Michael adduct was 97 : 3 e.r. Using this methodology pregabalin was synthesized in three steps in overall 52% yield.

 Please wait while we load your content...
Please wait while we load your content...