Metal free sulfenylation and bis-sulfenylation of indoles: persulfate mediated synthesis†
Abstract
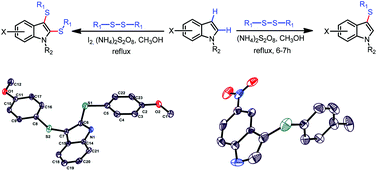
A method which avoids metal and halogen for the synthesis of 3-arylthioindoles from indoles and diaryl disulfides using ammonium persulfate in methanol has been presented. Moreover, double C–H sulfenylation of indoles at 2 and 3-positions has also been achieved using iodine and ammonium persulfate.

 Please wait while we load your content...
Please wait while we load your content...