A three step continuous flow synthesis of the biaryl unit of the HIV protease inhibitorAtazanavir
Abstract
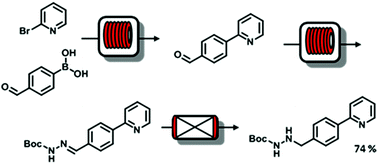
The development of multistep continuous flow reactions for the synthesis of important intermediates for the
- This article is part of the themed collection: Recent Advances in Flow Synthesis and Continuous Processing

 Please wait while we load your content...
Please wait while we load your content...