Boron functionalization of BODIPY by various alcohols and phenols†
Abstract
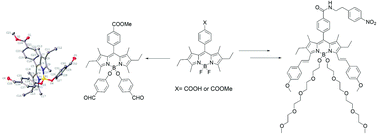
The synthesis of new B–O BODIPY derivatives functionalized with different alkoxy or diarylalkoxy derivatives is described. These compounds were synthesized from the reaction of different B–F BODIPY precursors with various alcohols and phenols, in the presence of AlCl3. Water-soluble dyes could be synthesized as well with this method, specifically by the introduction of polyethyleneglycol (PEG) groups. A photophysical study of the different compounds was performed, and showed that the B–O BODIPY derivatives exhibit rich fluorescence properties. Finally, the conjugation of the BODIPY core has been extended using two distyryl groups, hence providing NIR emitting BODIPY derivatives, in which one or two PEG groups have been anchored, making these systems very promising for future medical imaging applications.

 Please wait while we load your content...
Please wait while we load your content...