The relative hydrolytic reactivities of pyrophosphites and pyrophosphates†
Abstract
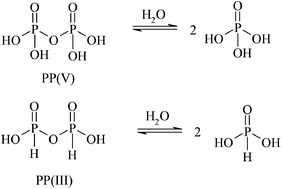
The pH-rate profiles for the hydrolysis of pyrophosphate (PP(V)) and pyrophosphite (PP(III), pyro-di-H-phosphonate) are a complex function of pH, reflecting the different ionic species and their relative reactivities. PP(III) is more reactive than PP(V) at all pHs and only PP(III) shows a

 Please wait while we load your content...
Please wait while we load your content...