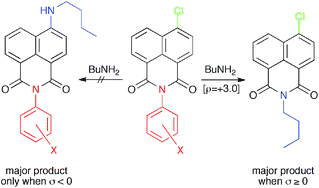
The heterocyclic ring of 4-substituted-1,8-naphthalimides is NOT inert to nucleophilic attack, contrary to earlier reports†
Abstract
The heterocyclic ring of N-aryl-4-chloro-1,8-naphthalimides, reported to be resistant to nucleophilic attack, reacts with

 Please wait while we load your content...
Please wait while we load your content...