An efficient coupling of N-tosylhydrazones with 2-halopyridines: synthesis of 2-α-styrylpyridines endowed with antitumor activity†
Abstract
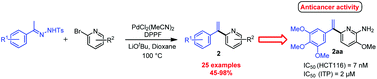
The synthesis of 2-α-styrylpyridines has been carried out by using the coupling of polyoxygenated N-tosylhydrazones with various 2-halopyridines. We demonstrated that the use of a catalytic amount of PdCl2(MeCN)2 in combination with a bidentate

 Please wait while we load your content...
Please wait while we load your content...