Specific nucleophile–electrophile interactions in nucleophilic aromatic substitutions†
Abstract
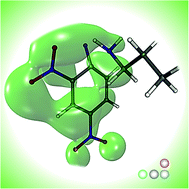
We herein report results obtained from an integrated experimental and theoretical study on aromatic nucleophilic substitution (SNAr) reactions of a series of

 Please wait while we load your content...
Please wait while we load your content...