Fluoride-catalyzed nucleophilic addition of PhSCF2SiMe3 to anhydrides: synthesis of γ-difluoromethylated γ-lactams†
Abstract
PhSCF2SiMe3 (1) underwent fluoride-catalyzed nucleophilic addition to the carbonyl group of anhydrides to provide the corresponding γ-difluoro(phenylsulfanyl)methyl γ-lactols, which were employed for the synthesis of γ-difluoromethylated γ-lactams.
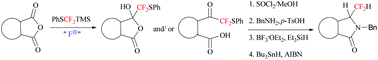

 Please wait while we load your content...
Please wait while we load your content...