Synthesis and biological evaluation of triazole-containing N-acyl homoserine lactones as quorum sensing modulators†
Abstract
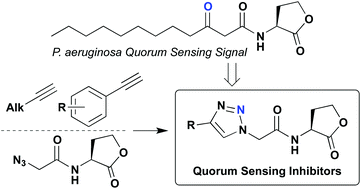
Many bacterial species are capable of assessing their local population densities through a cell–cell signaling mechanism termed quorum sensing (QS). This intercellular communication process is mediated by small molecule or

 Please wait while we load your content...
Please wait while we load your content...